Have you ever found yourself saying, “My CIP takes too long” or “My CIP does not get our equipment clean enough”? If so, you’re not alone. Countless businesses struggle with CIP systems that underperform. But here’s the good news – we can help.
With 18 years of experience designing, installing, and optimizing CIP systems from coast to coast, we literally wrote the book on CIP.

Are you tired of wasting money on cleaning expenses in your manufacturing plant? Look no further than our “CIP Basics” Book! As the experts in Clean In Place (CIP), we understand the hidden cost-saving opportunities that many plants overlook. In fact, we literally wrote the book on it.
Many individuals approach us after working with several people who have failed to solve their problems. This is quite understandable because finding sanitary process engineers with extensive industry experience can be quite challenging. We have witnessed projects experiencing delays of up to 24 months because a consultant who lacked sanitary process experience was hired to solve a cleaning issue.
At DeJong Operations Management & Consulting LLC, we ensure that the problem is solved correctly the first time. We possess the necessary experience to implement proven practices in your plant, which will result in bacterial loads being within the acceptable range, peak production efficiency, and a much shorter implementation period.
Clean in Place (CIP) is the process by which process piping, tanks, lines, and other sanitary process equipment are cleaned without disassembly or removal from the process lines. It involves using a combination of turbulent flow, chemical, and (usually) hot water to clean equipment interiors where product contact occurs. This method is designed to promote repeatability and reduce errors. Typically, implementing a good CIP program will reduce downtime and increase production capacity.
DeJong Consulting is a widely recognized expert in CIP design, CIP consulting, and CIP retrofitting – as well as a CIP skid manufacturing company.
A clean-in-place system typically pushes rinses and chemical cleaning washes through an existing process system, removing product residue without requiring equipment removal or disassembly. It’s a proven process that assists in reducing human error and can automate hygienic best practices.
Clean-in-place systems increase the consistency of a process line’s cleaning process. The speed and consistency offered by CIP often make it easier to increase the number of weekly cleaning cycles while decreasing the downtime required for cleaning processes.
Thanks to technological advances, clean-in-place technology is now more accessible to sanitary process facilities. Additionally, added customization and automation are now available. If you’ve previously discounted using them, it’s time for a second look.
Often, clients come to us after having previously installed CIP systems that do not deliver the time savings that they had expected. An effective CIP system uses multiple key processes that must work well with one another to deliver a proper end-cleaning result.
Even minor errors in the system or implementation can add hours of runtime. Often, we consult on these systems to optimize and improve runtimes.

Minimizes Mistakes: An automated clean-in-place system reduces the risk of error associated with a human-controlled cleaning process. The advantage of machine cleaning is that it tends to do the same cleaning process on every cleaning cycle. This typically means greater cleaning efficiency and a lot less human error.
Reduced Maintenance: Since much of this process is now automated, past processes of disassembly by highly-trained technicians are no longer required. This reduces both downtime and skilled labor costs. Additionally, there tends to be a lot less recurring damage to process equipment if it can remain in line for the cleaning process. Every disassembly adds wear and tear, so CIPable process equipment tends to last longer.
Employee Safety: Employees will usually be less exposed to harmful cleaning solutions and fumes. If process equipment doesn’t require removal, there are often major reductions in climbing hazards, slip-and-fall risks, and even (sometimes) reductions in confined space permitting. Additionally, lock-out/tag-out procedures are often reduced due to the reduction in process equipment that requires manual intervention.
Increased Production Time: Since no disassembly is needed, the cleaning cycle can be timed, accurate, and short. In some cases, we have found that the addition of effective CIP equipment has reduced the previously manual cleaning methods’ time investment by as much as 80-90%. This, of course, is highly situational, but we almost always see a reduction in time investment when switching to an automated CIP cleaning method.
Safety Standards: The reliable, repeatable, and regular cleaning intervals reduce contamination and promote consistent product quality. It also tends to reduce the variables in play. So, in the event of residual soils, bacterial growth, or other contaminants, the offending source tends to be much easier to identify and eliminate.
Utility Savings: Only the minimum required water and energy are used in a pre-calculated amount. The wastewater burden can often be reduced. In some cases, the CIP systems can be designed to neutralize chemical solutions before going to drain – helping to reduce the cleaning chemical pH that would normally cause conflict with municipality wastewater services. Possibly of greatest importance is that the usage rate of the cleaning chemicals themselves is often greatly mitigated by modern, automated CIP systems.
We have helped install CIP systems in Food, Biotech, and other Sanitary piping situations.
CIP is an automated clean-in-place system for sanitary food processing lines. The first CIP system was developed in the 1950s by Dale A Seiberling. Before that, all systems were disassembled and cleaned manually through physical scrubbing. This method has both high labor costs and also requires a greater downtime of the production line.
In the 1960s, the FDA began creating greater restrictions. Today, the process is highly standardized and repeatable, and it is a key component of modern food safety. Many existing Clean-Out-of-Place (COP) systems can be converted to clean-in-place. These conversions will often raise the productivity of a site while also decreasing food safety concerns.
In processing facilities, the HACCP (Hazard Analysis Critical Control Point) is a management system for analyzing and controlling biological contamination risks. CIP systems tend to support goals established by HACCP.
Additionally, CIP can contribute greatly to reducing chemical exposure risk by eliminating handling points that might typically come into contact with sanitizing solutions, caustic soda (or other caustic washes), or any other chemical solutions associated with the cleaning.
There are several key elements that we customize to your specific situation. In 1959, Chemical Engineer Herbert Sinner provided the 4 cleaning factors: Time, Action, Chemical, and Temperature, commonly referred to as TACT.
Time: This is the time that a surface area must be exposed to cleaning fluid to remove all product soil effectively.
Action: Impingement action on a surface. This can be the velocity of flow or spray design. It must, however, be turbulent flow while in line, requiring careful control of velocity.
Concentration & Conductivity: hemicals start with the chemistry of the specific soil. An effective chemical choice acts directly against the soils in question. Conductivity is the process by which the chemical percentage is verified on dosage or on return to the originating system.
Temperature: Temperature is an important consideration depending on the type of soil being removed from the product. This influences overall energy costs.
The Sinner’s Circle is based on the idea that all four factors work together with one another. Increasing the effectiveness of ANY of the four factors typically decreases the load on the other three. Automated CIP addresses all four simultaneously in an effort to reach a ‘best case’ scenario in terms of time, chemical use, energy use, and overall efficaciousness.
Our specialty is in upgrading existing plants to clean-in-place capability so that production can be increased within the existing facility footprint. To achieve this, our engineers ensure that these four factors are provided in sufficient quantity to every piece of the production line, regardless of flow or volume changes.
Generally, a typical CIP cycle consists of some combination of the below eight steps.
Pre-rinse water
Heated caustic wash (detergent or caustic solutions)
Intermediate Rinse (rare)
Acid wash cycle (rare)
Intermediate Rinse (rare)
Sanitizing rinse (situational)
Final Rinse
System Drain
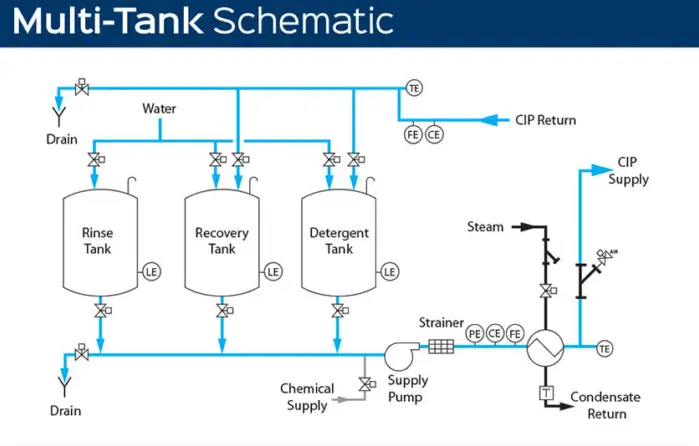
Each step is specifically calculated to create a reliable cleaning system. For example, fluid flow must be at the correct speed to create the proper amount of turbulent flow. Heat and detergent amounts are two essential pieces that determine how long it will take to complete the cleaning cycle. The final factor is the required time.
The most typical CIP cycle consists of pre-rinse, caustic wash, and post-rinse. All the other bells and whistles are possible but will be driven by what each specific process needs to ensure that the interior surface is clean throughout your process piping. DeJong’s process is that we start from the simplest solution and work our way to the required complexity to solve cleaning problems with the least steps possible.
There are several proven cleaning solution types.
Pre-Rinse: The primary goal is the removal of as much remaining soil from the process lines as possible. This step also provides a non-chemical integrity test of the CIP flow path before the chemical is introduced. Additionally, this step to remove residue helps to increase the effectiveness of the cleaning chemicals that will follow, which helps reduce overall chemical usage.
Product Recovery: In many cases, product recovery may precede this step or run in tandem with it. Water push, air-blow, pigging, or other methods can be used if the product is worth recovering.
Caustic Wash: This will often be a heated sodium hydroxide (or similar caustic base solution) wash that removes organic compounds. In many applications, it is part of the process to evaluate potential cleaning agents and either select an effective caustic solution from existing off-the-shelf available options or work with a chemical manufacturer to create a custom formulation. The important notes here are that the chemical concentration and formulation effectively neutralize the soils that remain on the process equipment surfaces after the pre-rinse. Additionally, this wash is usually very hot – most often, we see washes have the greatest effect between 160-200F.
Acid Wash: The acid wash neutralizes the system’s pH and dissolves the mineral scale. This typically applies to milk stone applications (in dairies) or in other mineral-laden processes. Another normal usage for an acid wash would be in more caustic environments that require regular re-passivation of the stainless steel piping and processing equipment product conduct surfaces.
Sanitizing Rinse (may be used instead of Final Rinse): If a system will be left for any extended period of time before the next production process, it is often current good manufacturing practice to include Peracetic acid (also known as peroxyacetic acid, or PAA) in the final rinse to prevent bacterial buildup in any water that the system retains after the CIP circuits are completed.
Final Rinse Water: Residual cleaning agents must be removed prior to the line resuming operation. In many CIP systems, this final rinse water is recaptured and will be heated and used for the pre-rinse of the next CIP regime, given that some caustic or acid will be recaptured on the first return of the rinse. Additionally, a reclaim process reduces the rinse water usage by roughly half (in most cases) and gives a way to get more “mileage” out of CIP cycles without adding to your cleaning chemical costs.
Chemical companies are essential in identifying the proper chemicals to break down your specific soil. They can be a critical resource in providing formulations for a CIP system. The downside of working with a chemical company is that they are paid by the gallon. As the saying goes, ‘To a hammer salesman, everything looks like a nail.’
While a chemical company is a vital part of an effective cleaning regimen, most CIP problems will require a company with more available solutions than simply the chemical formulation of the caustic or acid wash.
A good CIP system should have a repeatable recipe designed for effective cleaning. The best design for cleaning systems is one that allows operators to adjust the recipe as the plant changes.
Process plants tend to have a constant stream of small changes. Examples are things like processing equipment that is retired due to obsolesce or failure, changes to recipe or formulation, or the addition of new process circuits. In any of these cases, it’s likely that the CIP formula will need to be adjusted. The CIP process needs to be able to grow and change as the plant does.
As outside consultants, we are not paid for the amount of chemicals used. We often find efficiency and reduction usage opportunities. We usually find areas of optimization to minimize carbon footprint, wastewater burden, and chemical costs.
We also evaluate your CIP cycle (or manual cleaning) to determine if you’re effectively using rinse water for pre-rinse and post-rinse, look at your chemical solutions, and evaluate any cleaning agent that you may be using.
Equipment evaluation is key as well. During this process, we check the spray devices in your storage tanks, test interior surfaces on valves and pumps as needed, and evaluate your system design to see if current good manufacturing practices are being followed by your sanitation program.
Mix vessels can also be evaluated from drawings or in person to determine if there are shadowing challenges or vessel design constraints that might require more powerful spray devices.
If you’re trying to reduce CIP time in your processing plants, change the way your mix vessels are cleaned, or look at stabilizing food production that’s being interrupted by unexpected cleaning results, we can help.
If you have spray dryers, conveyor lines, or other equipment that might normally be difficult to CIP, we have a long and proven track record of finding ways to solve even the most complex industry cleaning problems. Your CIP system will revolve around the CIP skid. We design and build CIP skids to control the temperature, flow, and system of the CIP system.
Generally, a CIP system works best when designed as a modular system. This provides more flexibility for future plant growth while requiring less up-front cost and delays than a stick-built system requires.
If you want to know how to implement a recovery system (or even which recovery system style to choose), we can help you reduce energy consumption and rescue products from unnecessary loss.

DeJong Operations Management & Consulting LLC has a proven track record of helping clients from diverse industries maximize their return on investment and streamline their operations.